28+ calculate van t hoff factor
The greater the deviation from the ideal behaviour the lower is the Van t. Web First lets start by figuring out what you would expect the vant Hoff factor i to be for sodium phosphate Na_3PO_4.
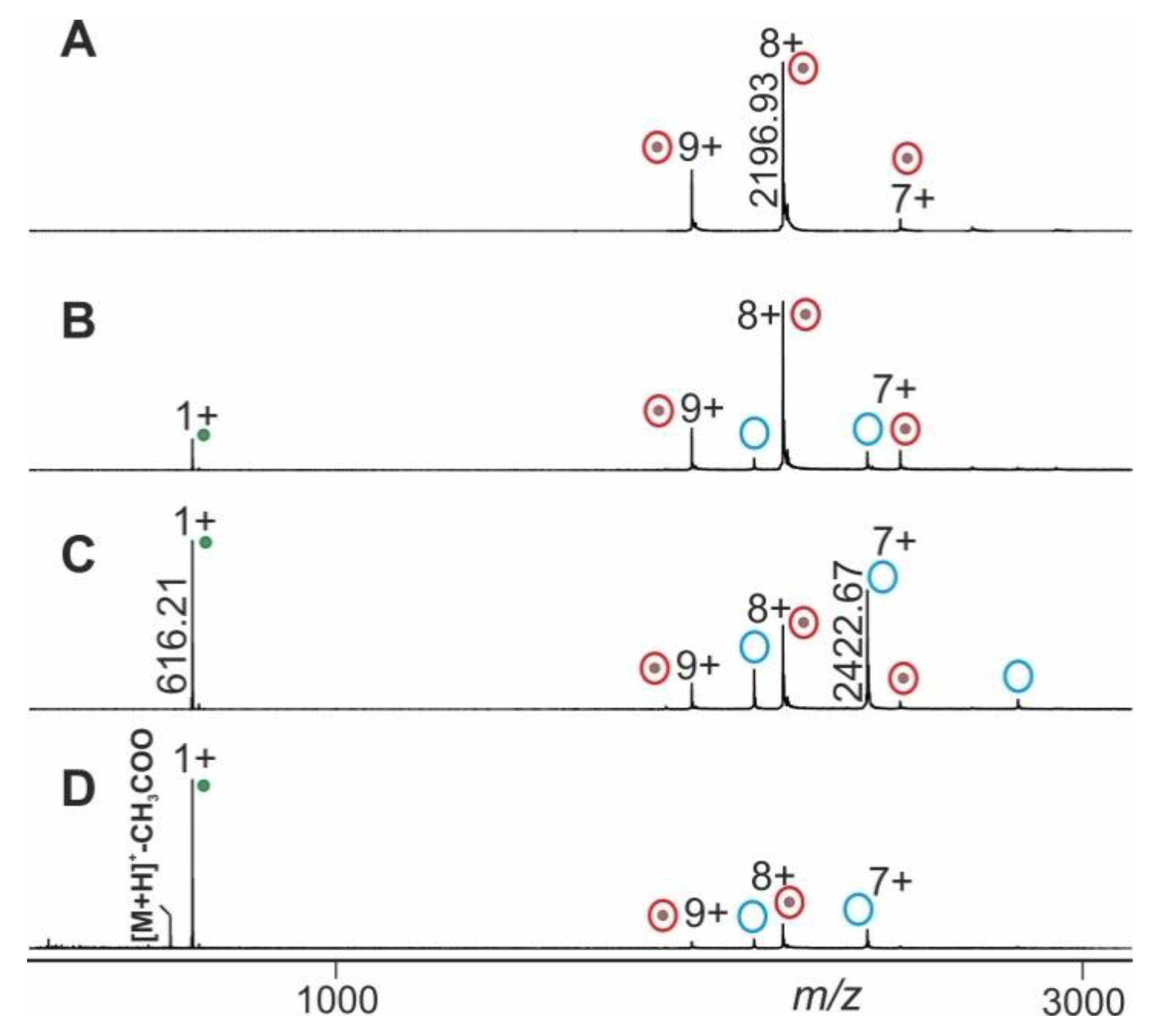
Molecules Free Full Text Mass Spectrometric Analysis Of Antibody Epitope Peptide Complex Dissociation Theoretical Concept And Practical Procedure Of Binding Strength Characterization
Notice that i is a property of the solute.

. The vant Hoff factor i is the number of particles formed in a solution from one formula unit of solute. Web Step 1. A 0126 M ionic solution has an osmotic.
Web For an aqueous solution of HF determine the vant Hoff factor assuming 0 ionization. Web The van t Hoff factor is the ratio between the actual concentration of particles produced when the substance is dissolved and the concentrationof a substance as calculated from. Revised equations to calculate the effect of ionization are.
Web To be quantitative we introduce the vant hoff factor i. Here we will use ideal van t Hoff factors. Web The actual van t Hoff factor is thus less than the ideal one.
Youll get a detailed solution from a subject matter expert that helps you learn core concepts. Web Calculating the Vant Hoffs Factor using the Osmotic Pressure Number of Moles Temperature in Kelvin Ideal Gas Constant and Volume. As you know the vant Hoff factor.
Web One of the most common formulas used to calculate the value of the Vant Hoff factor is i moles of particles in solutionmoles solute dissolved. I actual number of particles in solution after dissociation number of formula units initially dissolved in. Web This problem has been solved.
Web Equation for calculate vant hoff factor is i actual number of particles in solution after dissociation number of formula units initially dissolved in solution Vant Hoff Factor. The Van t Hoff plot which is derived from this equation is. Determine the number of moles q of released ions.
Web The Van t Hoff equation in chemical thermodynamics relates the change in the equilibrium constant Keq of a chemical equilibrium to the change in temperature T given the. Web Vant Hoff Factor i Calculated Molar MassObserved molar mass So Observed or experimentally obtained molar mass Calculated Molar MassVant Hoff Factor i 23. 𝑖 For the same solution determine the vant Hoff factor assuming 100 ionization.
Web The Vant Hoff factor is therefore a measure of a deviation from ideal behaviour. Analyzing the formula for salt we have index 1 in Fe and index 3 in Cl so the number of moles of ions. Web To compute for the boiling point elevation three parameters are needed and these parameters are Vant Hoffs Factor i ebullioscopic constant Kb and Molality.
Vant Hoff Factor for the. Get Help with Homework Math. Web The Van t Hoff equation has been widely utilized to explore the changes in state functions in a thermodynamic system.

Solved 1 Calculate The Van T Hoff Factor Measured Of Na 3po 4 In A 0 40 M Solution Whose Freezing Point Is 2 6 C K F 1 86 C M 2 Propyl Alcohol C3h7oh
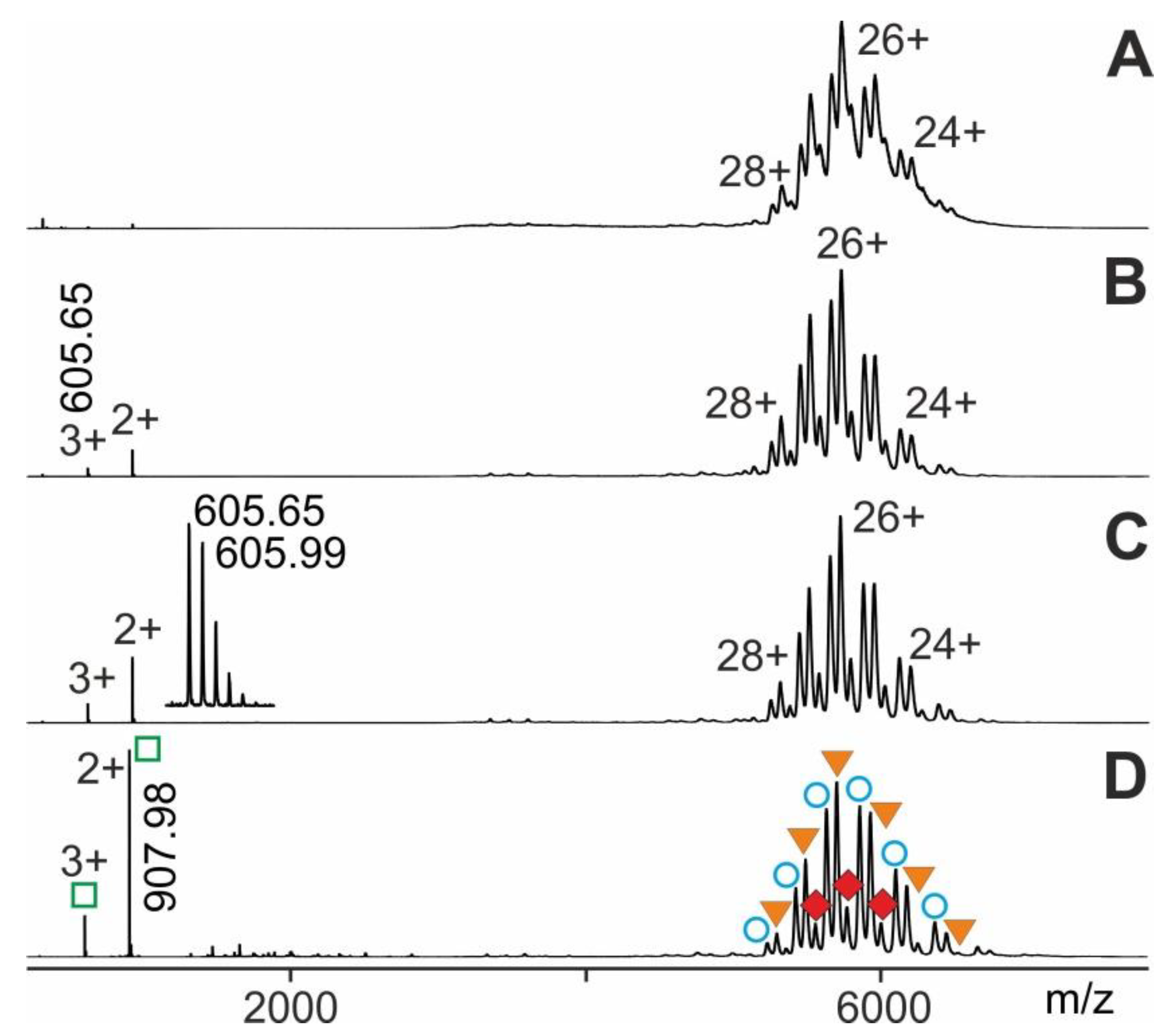
Molecules Free Full Text Mass Spectrometric Analysis Of Antibody Epitope Peptide Complex Dissociation Theoretical Concept And Practical Procedure Of Binding Strength Characterization

Chem 201 Calculating Actual Van T Hoff Factor Youtube

Thermodynamics Of Cement Hydration Pdf Cement Scanning Electron Microscope

Pdf Salivary Gland Organoid Culture Maintains Distinct Glandular Properties Of Murine And Human Major Salivary Glands
The Van T Hoff Factor

Lectures On Geochemical N1 Erpretation Of
How To Calculate The Van T Hoff Factor Of A Solute Quora

Aleks Calculating And Using The Van T Hoff Factor For Electrolytes 1 Of 2 Youtube

Chapter Wise Dpp Sheets For Che Disha Experts 2 Pdf

Solvent Drives Switching Between L And D Metal Center Stereochemistry Of M8l6 Cubic Cages Journal Of The American Chemical Society

The Van T Hoff Factor Definition And How To Calculate It

The Van T Hoff Factor Youtube

Chem 201 Calculating Actual Van T Hoff Factor Youtube

Chem 201 Calculating Actual Van T Hoff Factor Youtube
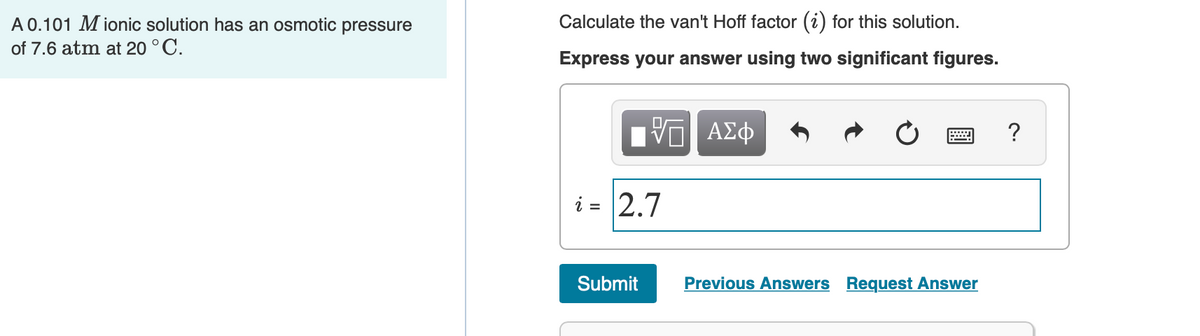
Answered Calculate The Van T Hoff Factor I For Bartleby
The Van T Hoff Factor